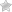Ammonia formula 🥝 what is the composition of ammonia

Is ammonia and ammonia the same thing? They are widely used in various fields of human activity. Some people think that ammonia is ammonia, but in reality it’s not at all what any chemist will prove to you. From this article you will learn what ammonia and ammonia are, and also what is the difference between them.
to contents ↑History of ammonia and its use in chemistry
How to make ammonia? The ammonia salt has been known to the Romans since very ancient times. The Romans collected in the territory of ancient Libya deposits of this substance near the temple of Amun.
The ammonia, as a form of ammonium chloride, was of great importance for Muslim alchemists in the 8th century. The first mention of this substance is found in the treatises of the Arab chemist Jabir ibn Hayyan, as well as in the works of 13th century European alchemists - when Albert the Great mentioned this element.
Ammonia was used not only for certain ceremonies, but also for changing the shade of plant-based dyes. Vasily Valentin in the 15th century proved that NH4Cl can also be obtained by the action of alkaline substances on ammonium chloride.
A little later, ammonium chloride began to be obtained by distillation of horns and hooves of cattle (oxen), and the neutralization of the obtained carbonate was carried out with hydrochloric acid. Due to this, the tool began to be called the "spirit of the deer horn."
to contents ↑The history of ammonia
Ammonia was first obtained in 1774 by the English chemist Joseph Priestley. The gas discovered by him was called “alkaline air”, since the chemist, and at the same time the priest, could not determine the chemical composition. Only 11 years later (in 1785) Claude Louis Bertollet, a famous French chemist, determined the chemical composition of the gas and called it ammonia (NH3).
There are two versions about why gas was named that way:
- one is associated with the name of the god of the ancient Egyptians, Amon;
- the second - with the sound of an oasis in the region of North Africa - “Ammon”.
According to the first version, people worshiping the god Amon, during the ceremony, sniffed ammonia.
Important! The chemical formula of ammonia is NH4Cl. When heated, it releases ammonia, so the gas having the chemical formula NH3 was called ammonia.
According to the second - in the oasis of Ammon, which is located at the crossroads of caravan routes, due to the presence of a huge number of pack animals, products of their vital activity accumulated. Under hot climates, urea tends to decompose quickly and produce a gas called ammonia.
Which of the two versions is reliable and correct is still unknown.
to contents ↑What is ammonia and ammonia?
Even judging by the history of occurrence, ammonia and ammonia are 2 different substances. The most important difference between these two chemical compounds is that under the same conditions they have a different state of aggregation:
- ammonia is a liquid;
- ammonia is a gas whose liquefaction occurs at a temperature of -33 C.
Important! Both substances have a characteristic unpleasant odor.
Many people think that ammonia is just a solution of ammonia in water. For domestic needs, it is often obtained this way, but the real process of ammonia formation from ammonia is longer and has two stages:
- Ammonium hydrate is formed from ammonia.
- When dissolving the resulting hydrate with water, it is precisely the composition that is called ammonia.
Thus, the opinion that ammonia is ammonia is wrong.
Important! In enterprises where ammonia is used, master refrigerators do not particularly bother themselves with complex chemical formulas. They act very simply: they lower the hose with ammonia into a bucket of water and at the same time get the amount of ammonia solution they need. The maximum saturation of the solution is determined by them by ear: as soon as specific clicks begin - that's it, gas is shut off, the product is ready.
The difference between these 2 substances is not only in chemical formulas, but also in applications. Ammonia is one of the most important products used in the chemical industry. This gas has found its application in this way:
- Production of ammonia.
- Construction (included in anti-frost additives).
- Production of polymers, soda and nitric acid.
- Fertilizer production.
- The manufacture of explosives.
- It is used to maintain the stable operation of various household and industrial equipment as a refrigerant.
Important! Ammonia is used in a narrower range of directions. Mostly - in medicine as an antiseptic, as well as in everyday life: many housewives remove stains from clothes of various origins with this solution.
Ammonia is often used in everyday life along with other similar means. How exactly and how effective in any situation - read all aboutammonia and hydrogen peroxide!
to contents ↑Instructions for use
Ammonia is available in several forms:
- glass bottles from 10 to 100 ml for external use;
- 1 ml ampoules (10% aqueous ammonia solution).
Store the drug in dark, cool places. The ampoule is valid for 5 years, for vials - 2 years.
Indications for use of the drug
- Inhalation - to stimulate breathing, as well as to quickly remove a person from a fainting state.
- Outwardly - during the treatment of hands in surgical practice and for disinfecting the skin in order to eliminate itching after bites of various insects.
- Inside - exclusively as an emetic.
Important! Remember that ammonia is not ammonia and cannot be used in the same way. A pure ammonia compound can cause significant harm to human health.
Pharmacodynamics of the drug
When inhaled ammonia, the drug acts on the receptors of the upper respiratory tract. In this case, the reflex respiratory center is involved. Also, the drug reflexively affects the work of the heart and vascular tone.
During the use of the drug, an emetic center is excited inside, due to which it becomes possible to empty the digestive system from toxins.
When applied to the skin of the drug, a distracting effect through the skin receptors is carried out. The drug suppresses the focus of excitation, reduces pain and muscle tension, relieves tissue spasms. At the site of contact with the drug, skin receptors erupt, which provokes the release of active substances. Due to this, vasodilation occurs, the process of tissue regeneration and nutrition is accelerated, the outflow of metabolites is normalized.
Pharmacokinetics
Ammonia is rapidly excreted by the bronchial glands and lungs.
Application rules
In medical practice, such alcohol is often used to have an irritating effect on the receptors of the nasal mucosa during fainting, as well as alcohol poisoning. To use the drug correctly, follow these instructions:
- To normalize breathing and bring a person “to feelings” with fainting, bring a piece of cotton moistened with ammonia solution to the patient’s nostrils.
Important! Keep cotton wool no closer than 5 cm from the nose so that there is no burns when the drug comes in contact with the skin.
- In case of alcohol poisoning, you can give the victim drink ammonia, but only in diluted form.Proportions: 5-6 drops per glass of water.
- As an expectorant, use ammonia anise drops. This combination preparation includes ammonia solution, ethyl alcohol and anise oil. Recommended daily dose:
- For adults - up to 15 drops (no more than 5 drops twice or thrice a day).
- Children under 1 year old - a maximum of 1 drop, up to 2 times a day.
- To provoke vomiting, use the drug in diluted form: 5-7 drops of the drug in half a glass of water.
Important! On a diluted preparation, when taken orally, it causes burns to the esophagus.
- To wash hands in surgical practice, also use the product diluted: 25 ml of the drug in 5 liters of boiled warm water.
- An ointment made of ammonia and lanolin in equal amounts is used to stop unpleasant symptoms after insect bites.
Precautionary measures
- Inhalation of vapors of a solution of ammonia, ammonia in large quantities can cause respiratory arrest and heart rhythm disturbance.
- If the drug is taken in large concentrations by mouth, then the following symptoms will appear: abdominal pain, indigestion, vomiting, cramps.
- If an overdose occurs during inhalation, a runny nose, cough, swelling of the larynx, and respiratory arrest are possible.
- With external use, an overdose is fraught with burns.
Important! The lethal dose of ammonia solution is 10-15 g.
First aid for poisoning:
- In case of ammonia poisoning, the victim should be taken out to fresh air, throat, nose, mouth and rinse thoroughly with water. For greater effectiveness, add glutamic or citric acid to the water.
- If the drug disappears into an open area of the body, rinse thoroughly with water and cover the damaged skin with a bandage. It is not recommended to use any ointments within 24 hours, and subsequently carry out the therapeutic treatment the same as with thermal burns.
- If ammonia, high concentration ammonia, has entered the digestive tract, rinse your stomach thoroughly. Let the affected person drink some protein from eggs, a spoonful of vegetable oil, a glass of milk. If possible - give an enema.
- If ammonia splashes into your eyes, rinse immediately with running water. After that, apply petroleum jelly or olive oil to the affected area on the skin and instill eye drops with 0.5% dicaine solution, and if necessary, cover your eyes with a blindfold.
to contents ↑Important! In case of poisoning of any degree, contact a medical institution for qualified assistance and to prevent serious negative consequences from ammonia, ammonia for your health.
The use of ammonia in everyday life
Ammonia water is used in gardening, as well as in everyday life, as a universal cleaning agent. It is used in the fight against aphids, for processing cultivated plantings on the site from the onion fly and for feeding plants. Use ammonia (ammonia) as follows.
Recipe number 1 - from aphids:
- 4 tbsp. l the drug is diluted in 20 liters of water. The amount can be changed based on such a concentration for the desired volume.
- Add a little washing powder to the solution (for better adhesion).
- Use a solution to spray plants when fighting aphids.
Ammonia and ammonia are excellent stain removers. Check it out right now by following the tips from the suggested articles.
Recipe number 2 - fertilizer
Dissolve 50 ml of the drug in 4 l of water. Use the solution as a top dressing for garden and indoor plants.
Important! This tool will also allow you to get rid of mosquitoes and midges.
Recipe number 3 - for watering onions
- Dilute 1-2 tbsp. l ammonia in a bucket of water.
- Water the onion with the product - from the moment of planting until the end of June.
Recipe number 4 - gold cleaning
- Mix 2 tsp. ammonia with 2 glasses of water.
- Add to the solution 1 tbsp. l any detergent.
- Place gold jewelry in the cleaning solution for 1-2 hours.
- After processing, wash the products in water and wipe dry with a cloth.
Important! For cleaning gold, this tool can be used in another way:
- Add 0.5 tsp. ammonia in 100 ml of water.
- Add 30 ml of hydrogen peroxide and ½ tsp to the solution. liquid detergent.
- Place the decoration in the prepared solution for 15 minutes.
- After processing, rinse the gold with water and wipe dry with a cloth.
Recipe number 5 - cleaning silver
- Dilute ammonia with water - proportions 1:10.
- Leave the silver product in solution for several hours.
- After cleaning, rinse the silver under water and wipe it with a soft cloth.
to contents ↑Important! For regular silver cleaning, use a soap solution with a small amount of ammonia.
Stock footage
Ammonium hydroxide is an important source of nitrogen for all living systems. Nitrogen is present in large quantities in the atmosphere (more than 75%), but not many living creatures are able to use it, because nitrogen is necessary for the biosynthesis of amino acids, which are the building blocks of protein. We hope that this article has helped you evaluate the usefulness, properties and advantages of ammonia.